Using quantum calculations and the first molecular model of the edges of gamma alumina crystallites, a material widely employed in catalysis, teams from IFPEN and the CRMN revisited the NMR spectrum assignment established in the literature up until now. They discovered that the peaks observed on the spectrum corresponded to hydroxyl protons present on the edges.
Gamma alumina: secrets still to be revealed on an atomic scale
Aluminum oxide, or Al2O3, is a material that offers a broad range of applications in the field of heterogeneous catalysis, since it presents an intrinsic reactivity exploited to disperse the active phases (metal, sulfur) of catalysts. This is the case with so-called gamma (γ) alumina, for example, which is widely employed in industry and has therefore been the focus of many studies over several decades but which still conceals a number of secrets on an atomic scale.
Research recently conducted at IFPEN [1] in partnership with the CRMN (high-field nuclear magnetic resonance spectroscopy center in Lyon), within the framework of the CARMEN joint research laboratory and the ROAD4CAT Chair, uncovered a new aspect of the properties of gamma alumina, seldom mentioned in the literature until now.
NMR spectrum: presence of hydroxyls on alumina crystallites
Gamma alumina exists in the form of microscopic crystallites with finite dimensions in the range of tens of nanometers. The surfaces and edges of these crystallites are made up of Al and O atoms that present coordination defects compared to their counterparts located in the core of the material. To compensate for this, the atoms on the surface and the edges have a propensity to create new chemical bonds with water molecules, thereby creating hydroxyl groups (Alsurf-OH) that play a crucial role in the reactive properties of gamma alumina.
The NMR spectra of the solid’s 1H protons (800 MHz high-field NMR) were obtained for two gamma alumina samples that differed in crystallite morphologies and sizes. These spectra revealed that the hydroxyls (OH groups, the only H source in alumina) are present on alumina crystallites and that their concentration depends on the nature of the two samples.
A new quantum molecular model revisits spectrum assignments
Quantum calculations (DFT1) were used to revisit the NMR spectrum assignment in regard to previous proposals disclosed in the literature. It was shown that one of the main peaks observed on the spectrum corresponded to hydroxyl protons (monocoordinated) located on the edges of alumina crystallites [1].
This discovery was made thanks to the construction of the first quantum molecular model of the edges of gamma alumina crystallites. This model proposes a new atomic architecture for edges of this type in the presence of hydroxyls. Quantum calculations of the proton chemical shift reveal that these edge hydroxyls correspond to the NMR peak at δ=-0.1 ppm (figure). This new NMR spectrum peak assignment can be used to explain how the effects of size and morphology of the two samples studied impact the nature of the NMR spectrum.
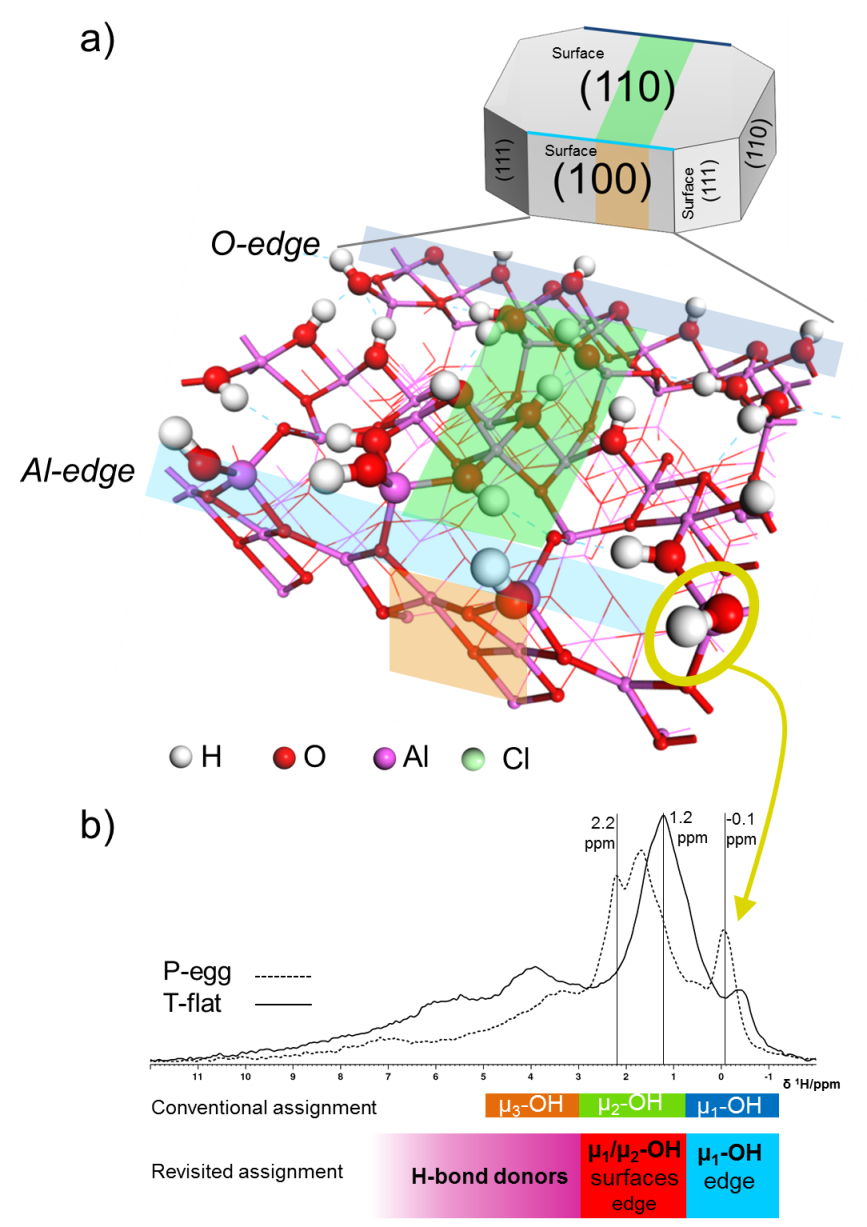
Figure: a) Gamma alumina crystallite and detail of the edge model ; b) Proton NMR spectra of the two aluminas studied (P-egg, morphology with numerous edges and T-flat with few edges), the assignment of these signals in the previously existing literature and the revisited assignment proposed in [1].
Other discoveries useful for catalysis
For the first time, this research also demonstrated for that chlorine, used to boost acidity for some catalytic applications, selectively exchanges the hydroxyls of these same edges, leading to the disappearance of the famous peak at δ=-0.1 ppm.
Moreover, this new approach makes it possible to simultaneously measure the quantity of active sites located on the edges of alumina crystallites, sites that may be crucial for catalysis.
By using other cutting-edge characterization tools (High-Resolution Electron Microscopy, in partnership with the IPCMS2), this research was recently extended to cover the crucial issue of supported active phases, such as platinum, highlighting the effect of crystallite edges on the stabilization of these metal nanoparticles [2].
1 Density-functional theory
2 Institut de Physique et Chimie des Matériaux de Strasbourg (Strasbourg Institute of Materials Physics and Chemistry
Scientific contact: Ana Teresa Fialho Batista
References:
[1] Beyond gamma-Al2O3 Crystallite Surfaces: the Hidden Features of Edges Revealed by Solid-State 1H NMR and DFT Calculations.
Batista, A. T. F. ; Wisser, D. ; Pigeon, T. ; Gajan, D. ; Diehl, F. ; Rivallan, M.; Catita, L.; Gay, A.-S.; Lesage, A.; Chizallet, C.; Raybaud, P.
J. Catal. 2019, 378, 140–143.
https://dx.doi.org/10.1016/j.jcat.2019.08.009
[2] Atomic scale insight into the formation, size and location of platinum nanoparticles supported on γ-alumina.
Atomic scale insight into the formation, size and location of platinum nanoparticles supported on γ-alumina.
Batista, A. T. F. ; Bazziz, W.; Taleb, A.-L.; Chaniot, J.; Moreaud, M. ; Legens, C.; Aguilar-Tapia, A.; Proux, O.; Hazemann, J.-L.; Diehl, F.; Chizallet, C.; Gay, A.-S.; Ersen, O.; Raybaud, P.
ACS Catal. 2020.
https://dx.doi.org/10.1021/acscatal.0c00042